Significance of Mycobacterium tuberculosis
Mycobacterium tuberculosis (Mtb), the causative agent of human tuberculosis (TB), is transmitted through aerosols and successfully replicates within alveolar macrophages by inhibiting macrophage maturation. Mtb has surpassed HIV/AIDS as the leading cause of death worldwide from a single infectious agent. In 2018, 10 million people were infected with Mtb resulting in 1.2 million deaths. The WHO estimates that globally 3.4% of new cases and 18% of previously treated cases were multidrug resistant TB (MDR-TB) in 2018. Thus, there is an imminent need to develop chemotherapeutic strategies to prevent TB disease progression.
Significance of Iron availability during TB disease progression
Mtb is dependent on iron acquisition to successfully colonize the human host. To limit Mtb infection, iron is sequestered in host binding proteins such as transferrin (Tf), ferritin, and lactoferrin (Lf); or in the form of heme within hemoproteins. Mtb secretes siderophores to acquire iron from Tf, ferritin and Lf, but the siderophores cannot access iron in heme or hemoglobin, which store more than 75% of host iron. Recently, it was shown that the necrotic centers of TB granulomas (infected macrophages) contain high concentrations of heme- and hemoglobin-sequestering proteins, possibly to limit access to heme iron. This observation is particularly relevant in the context of the Mtb life cycle because: 1) macrophages play a key role in the recycling of senescent and damaged erythrocytes for hemoglobin production in new erythrocytes and 2) Mtb resides and replicates within host macrophages. Thus, heme is a major iron source for Mtb during its life cycle within the human host, however we have no knowledge of the importance of heme in Mtb virulence because we lack a basic understanding of Mtb heme uptake mechanisms.
Heme acquisition by Mtb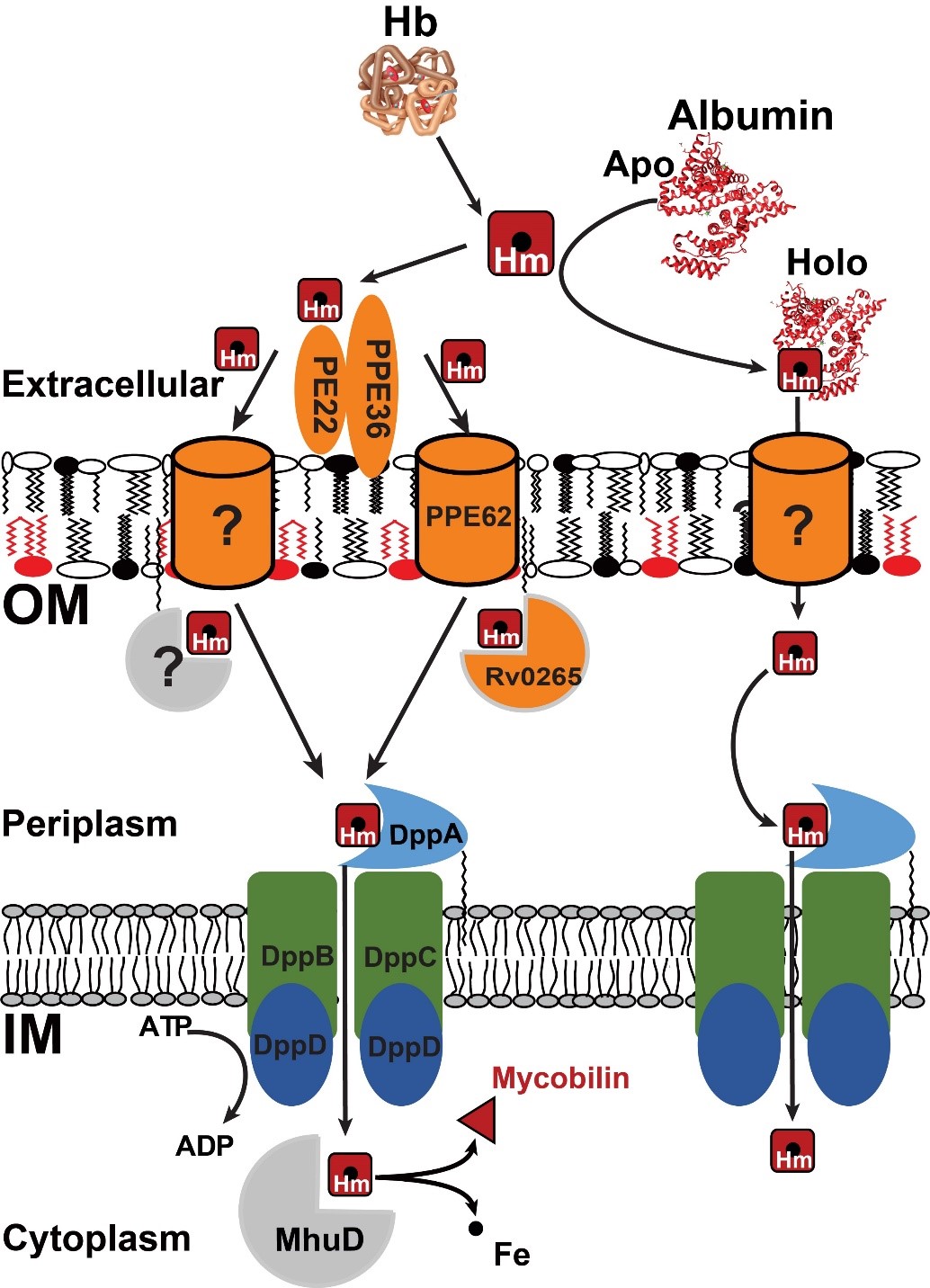
Our studies show that Mtb has at least two heme acquisition pathways. We discovered that in one pathway the outer membrane proteins PPE36 and PPE62 bind heme and are required for efficient heme utilization, and that the inner membrane Dpp transporter is essential for transporting heme into the cytoplasm. Importantly, we showed that mycobacterial PPE proteins are functional homologs of gram-negative β-barrel outer membrane proteins (OMPs) that are used for heme acquisition. This is significant because Mtb does not have any β-barrel OMPs. We also discovered a new and entirely different pathway where heme utilization is mediated by albumin. The existence of an albumin-heme pathway has profound implications in the context of iron availability to Mtb because albumin is the most abundant protein in blood and strongly binds heme. Thus, Mtb may use this pathway for heme acquisition when blood is availablen bloodstream.
Goals
Despite these recent discoveries, significant gaps remain in our understanding Mtb heme utilization. Our goals are to understand molecular mechanisms of Mtb heme uptake and to understand the exact biological relevance of heme utilization to Mtb virulence and disease progression.
